Corrosion of structural steel
The corrosion of structural steel is an electrochemical process that requires the simultaneous presence of moisture and oxygen. Essentially, the iron in the steel is oxidised to produce rust, which occupies approximately six times the volume of the original material. The rate at which the corrosion process progresses depends on a number of factors, but principally the 'micro-climate' immediately surrounding the structure.
[top]The corrosion process
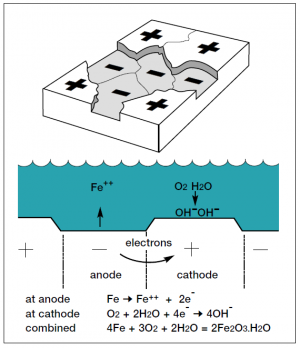
The corrosion of steel can be considered as an electrochemical process that occurs in stages. Initial attack occurs at anodic areas on the surface, where ferrous ions go into solution. Electrons are released from the anode and move through the metallic structure to the adjacent cathodic sites on the surface, where they combine with oxygen and water to form hydroxyl ions. These react with the ferrous ions from the anode to produce ferrous hydroxide, which itself is further oxidised in air to produce hydrated ferric oxide (i.e. red rust.) The sum of these reactions can be represented by the following equation:
Fe + 3O2 + 2H2O = 2Fe2O3H2O
(Steel) + (Oxygen) + (Water) = Hydrated ferric oxide (Rust)
However, after a period of time, polarisation effects such as the growth of corrosion products on the surface cause the corrosion process to be stifled. New, reactive anodic sites may be formed thereby allowing further corrosion. In this case, over long periods, the loss of metal is reasonably uniform over the surface, and this is usually described as 'general corrosion'. A schematic representation of the corrosion mechanism is shown (right).
The corrosion process requires the simultaneous presence of water and oxygen. In the absence of either, corrosion does not occur.
[top]Localised corrosion
Various types of localised corrosion can also occur but these tend not to be significant for structural steelwork.
[top]Bimetallic corrosion
When two dissimilar metals are joined together and in contact with an electrolyte, an electrical current passes between them and corrosion occurs on the anodic metal. Some metals (e.g. stainless steel) cause low alloy structural steel to corrode preferentially whereas other metals (e.g. zinc) corrode preferentially themselves, thereby protecting the low alloy structural steel. The tendency of dissimilar metals to bimetallic corrosion is partly dependent upon their respective positions in the galvanic series. The further apart the two metals in the series the greater the tendency.
Another aspect that influences bimetallic corrosion is the nature of the electrolyte. Bimetallic corrosion is most serious for immersed or buried structures, but in less aggressive environments e.g. stainless steel brick support angles attached to mild steel structural sections, the effect on the steel sections is minimal. No special precautions are required in most practical building or bridge situations. For greater risk situations, gaskets, sleeves and similar electrically insulating materials should be used. Alternatively the application of a suitable paint system over the assembled joint is also effective.
The tendency for bimetallic corrosion is also influenced by the relative surface areas of the cathodic and anodic metals (Ac/Aa). In simple terms, the greater the Ac/Aa ratio, the greater the tendency for bimetallic corrosion.
[top]General galvanic series
Anodic end (More prone to corrosion)
- Magnesium
- Zinc
- Aluminium
- Carbon & Low Alloy (Structural) Steels
- Cast Iron
- Lead
- Tin
- Copper, Brass, Bronze
- Nickel (Passive)
- Titanium
- Stainless Steels 430/304/316 (In the passive state)
Cathodic end (Less prone to corrosion)
[top]Pitting corrosion
In some circumstances the attack on the original anodic area is not stifled and continues deep into the metal, forming a corrosion pit. Pitting more often occurs with low alloy structural steels in continually wet conditions or buried in soil rather than those exposed in air. Hence, pitting corrosion is rarely encountered on typical modern steel buildings or bridges.
[top]Crevice corrosion
Crevices can be formed by design detailing, welding, surface debris, etc. Available oxygen in the crevice is quickly used by the corrosion process and, because of limited access, cannot be replaced. The entrance to the crevice becomes cathodic, since it can satisfy the oxygen-demanding cathode reaction. The tip of the crevice becomes a localised anode and high corrosion rates occur at this point.
[top]Corrosion rates
The principle factors that determine the rate of corrosion of steel in air are:
[top]Time of wetness
This is the proportion of total time during which the surface is wet, due to rainfall, condensation etc. It follows, therefore, that for unprotected steel in dry environments e.g. inside heated buildings, corrosion will be minimal due to the low availability of water. The requirement for the application of paints or coatings becomes unnecessary other than for appearance or fire protection purposes.
[top]Atmospheric pollution
The type and amount of atmospheric pollution and contaminants (e.g. sulphates, chlorides, dust etc.)
[top]Sulphates
These originate from sulphur dioxide gas produced during the combustion of fossil fuels, e.g. sulphur bearing oils and coal. The sulphur dioxide gas reacts with water or moisture in the atmosphere to form sulphurous and sulphuric acids. Industrial environments are a prime source of sulphur dioxide.
[top]Chlorides
These are mainly present in marine environments. The highest concentration of chlorides is to be found in coastal regions and there is a rapid reduction moving inland. In the UK there is evidence to suggest that a 2 kilometre strip around the coast can be considered as being in a marine environment.
Both sulphates and chlorides increase corrosion rates. They react with the surface of the steel to produce soluble salts of iron, which can concentrate in pits and are themselves corrosive.
Within a given local environment, corrosion rates can vary markedly, due to effects of sheltering and prevailing winds etc. It is therefore the 'micro-climate' immediately surrounding the structure, which determines corrosion rates for practical purposes. Because of variations in atmospheric environments, corrosion rate data cannot be generalised. However, environments can be broadly classified, and corresponding measured steel corrosion rates provide a useful indication of likely corrosion rates. More information can be found in BS EN ISO 12944-2[1] and ISO 9223[2]. Image does not exist
[top]References
- ↑ 1.0 1.1 BS EN ISO 12944-2: 1998, Paints and varnishes – Corrosion protection of steel structures by protective paint systems – Part 2: Classification of environments, BSI
- ↑ ISO 9223: 2012, Corrosion of metals and alloys – Corrosivity of atmospheres – Classification, International Standards Organisation
[top]Resources
- Steel Buildings, 2003, The British Constructional Steelwork Association Ltd.
- Chapter 12 – Corrosion Protection
[top]Further reading
- D.Deacon & R.Hudson (2012), Steel Designer’s Manual (7th Edition), Chapter 36 - Corrosion and corrosion prevention, The Steel Construction Institute.
- D.A. Bayliss & D.H.Deacon (2002), Steelwork Corrosion Control (2nd edition), Spon Press.
- PD 6484:1979, Commentary on corrosion at bimetallic contacts and its alleviation, British Standards Institution.
[top]See also
- Influence of design on corrosion
- Surface preparation
- Paint coatings
- Standard corrosion protection systems for buildings
- Metallic coatings
- Appropriate specifications
- Inspection and quality control