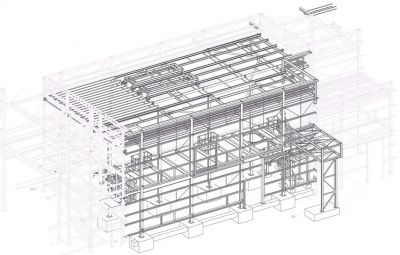
Cold Store and Purified Water Plantroom, GlaxoSmithKline, Barnard Castle
Article in NSC July/August 2021
Extension works
Leading Pharmaceutical company GlaxoSmithKline is continuing investment at its Barnard Castle site with rapid construction of a new steel-framed cold store and purified water plant extension.
Following on from the major investment in its manufacturing sites announced in 2016, GlaxoSmithKline (GSK) continues to invest in the Barnard Castle site to support New Product Introduction programmes and existing facilities. The latest investment includes construction of additional cold storage capacity and a new purified water plant room extension. Barnard Castle is one of GSK’s largest secondary manufacturing sites, employing around 1,000 people. The site supplies nearly half a million packs of products per day to 140 global markets. The recent major investment included Q Block, a new advanced aseptic manufacturing facility that will be utilised for delivery of GSK’s pipeline of new biopharmaceutical products.
The steel-framed structure that houses the cold store and purified water plant room extension measures 26m-long × 15m-wide × 11.2m-high and is connected to the existing Q Block structure. GSK Project Manager Lauren Du Bourg says: “The original plans for the building included the option for future cold store expansion, which would support capacity increase for the site’s biopharmaceutical new product pipeline.”
One of the main challenges for the site team, including steelwork contractor Border Steelwork Structures (BSS), was completing the erection programme in and around a functioning biopharmaceutical building. The main steel-framed facility was completed and qualified in 2019/20 and is currently undergoing a final performance qualification. “The steelwork installation required a 40t-capacity crane to deliver our complete package in a three-week programme,” explains BSS Contracts Director Stuart Airey. “It was a relatively small building extension, but safety was upmost priority working on a live GSK site. We had our own Contracts Manager onsite throughout the works who coordinated very well with the GSK site team, and this resulted in a very good outcome to the schedule.”
It was essential to prevent weather ingress to the existing building, consequently BSS began its work by stripping off the existing cladding and temporarily weatherproofing the three elevations of Q Block the new steel extension connected to. Steel connection modules were then carefully installed to allow the structures to be connected during the erection of the new extension. “Steel was chosen for this scheme because of is speed of construction and installation flexibility. Consequently, with minimal preparatory work, we have been able to simply bolt-on the extension to the existing structure,” says BakerHicks Project Director Tom Dickinson. Once the steel frame was installed, BSS’s work also included the installation of roof cladding, wall cladding and louvres. The site was then opened up to the other follow-on trades.
The new steelwork gains its stability from the existing steel-framed structure, as it connects to an existing frame on three sides, and along the longest 26m-long elevation it also includes a movement expansion joint. “In order to have continuity of movement, we had to have a dog-leg connection to the new perimeter columns that form a new continuing expansion joint,” adds BakerHicks Engineer William Bancroft. “Forming part of the cold room extension, a series of 9m-long columns connect to this new movement joint via a sliding connection. They are spaced at 1.2m centres as they support the roof cladding.”
Overall, the cold store is a single storey structure, and its frame is completed by further internal spans of 8.5m. However, the internal space is supplemented by additional floorspace in the adjoining existing structure. “The cold store will be utilised for storage of cold chain pharmaceutical products, including both biopharmaceutical and chemical drug products. The capacity demands on site are increasing as our new pipeline of biopharmaceutical products deliver a range of new medicines including key oncology treatments,” explains GSK Engineering Director Nigel Wood.
The building structure also includes a new purified water plant room located along the north elevation. This part of the structure accommodates a mezzanine level, with a floor formed with a galvanized metal grid system. This flooring was chosen as it offers a hard-wearing, safe, durable solution, while it can also be easily removed if the building was repurposed in the future. “The new purified water generation equipment replaces legacy equipment with improved generation capacity to ensure robust high quality water supply to our manufacturing operations and supports the ‘speciality ready’ status of the site for new product introduction for GSK,” adds Lauren Du Bourg.
GSK’s Barnard Castle extension is due to complete later this year.
| Structural Engineer | BakerHicks |
| Steelwork Contractor | Border Steelwork Structures |
| Main Client | GlaxoSmithKline |